Mike Wilson | 06 February 2024
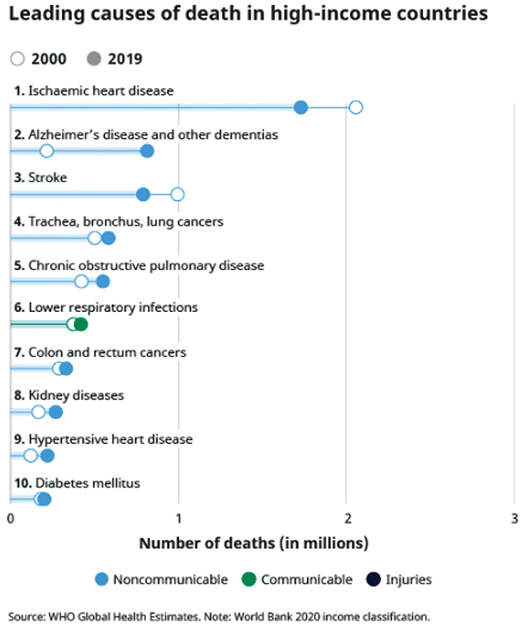
Alzheimer’s disease is one of the most common causes of death in the world. Usually a disease of older age, data suggests dementias, more broadly, are now the second or third leading cause of death in high-income countries, depending on whether cancers are all counted together or not. According to the WHO, Alzheimer’s recently overtook strokes and is behind only ischaemic heart disease.
Total deaths from dementias in high-income countries have almost quadrupled in the last 20 years, yet so far medical science has very few answers. This is not for want of effort. Scientists have dedicated vast amounts of time and money seeking ways to at least slow down, if not reverse or prevent, this degenerative disease.
This article looks at the recent emergence of the first drugs which may be able to modify the effects of this terrifying disease.
Hope at last
For decades, scientists have struggled to understand what causes Alzheimer’s and develop drugs which can arrest, or at least slow, its progress. The biological mechanisms causing this disease remain poorly understood, and there are probably several which contribute. However, of these, one dominates much recent Alzheimer’s research.
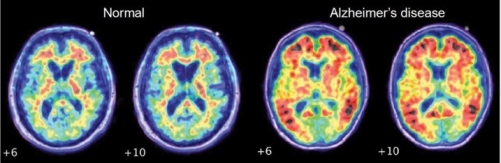
Many scientists believe that the disease is caused by aggregations of proteins in the brain. This theory, known as the ‘amyloid hypothesis’ states that strands of a protein, known as amyloid beta, clump together to form deposits known as amyloids. Over time, more proteins become stuck to these deposits, eventually forming plaques which are large enough to disrupt communication between cells in the brain. Immune cells are activated, causing inflammation and, ultimately, the destruction of brain cells.
If this is how Alzheimer’s develops, then it makes sense to try to slow its progress by tackling these amyloid deposits. Three new drugs have created headlines recently as potential ‘game- changers’ for people with Alzheimer’s, and this is exactly how each of them tackles the problem.
The first, aducanemab, was conditionally approved for use in the United States in 2021. (1) The drug was demonstrably able to reduce amyloid plaques in people using it, but failed to meet its primary endpoint of clinically improving patients’ symptoms such as memory loss. As such, its approval was controversial. Lacking evidence that it would make any noticeable difference to patients, understandably, many doctors refused to prescribe it. Medicare – federal health insurance for Americans aged over 65 – and regulators elsewhere, including the UK and EU, also did not approve the drug for use.
Next on the scene was lecanemab. This too showed it could reduce amyloid plaques in the brain - dramatically so, in fact. This time there was also more positive news for patient outcomes, with trials suggesting it slowed decline in memory and thinking by a modest 27% after 18 months of treatment. Given the low bar for approval set by aducanumab, it is perhaps unsurprising that lecanemab was fully approved in July 2023 in the USA.2 Regulators elsewhere continue to assess the trial findings to determine if wider approval will follow.
Very recently, donanemab became the third drug to show some success in this area. It again demonstrated good success at reducing the underlying plaques, with substantial reductions in measurable plaques seen in around 80% of trial participants.
Although its abilities to improve clinical outcomes, e.g. memory loss, for patients were again modest, it appears at least as impressive as lecanemab. Disease progression slowed by around one-third in one sub-group of patients who had lower levels of another protein called tau. This effect reduces significantly to 22% slowing if people with higher levels of tau are included. The drug’s approval in the US is pending but expected to be granted.
Tempering expectations
It is undoubtedly exciting that medical science is reaching a point where it can make inroads against this disease. However, we must note the limitations of these drugs. The most fundamental of these is that their impacts on the symptoms of Alzheimer’s remain unspectacular, and limited to those with early-stage disease. Focusing on donanemab, because it appears the most promising, its trials actually produced counter-intuitive outcomes.
On the one hand, the drug achieved statistically significant improvements in patient outcomes, meaning that it is highly likely that the group taking the drug fared better than the one taking a placebo, and that the drug was the cause of this difference.
However, clinical significance - such as cognitive changes in a patient that can be measured - is in doubt. Progression of Alzheimer’s is difficult to measure and more subjective than for many diseases. The disease is measured by administering tests seeking to determine patients’ abilities in areas such as word recall, orientation, memory and word-finding, among many others. On one test used in the trial, changes of at least 5 points on the scale are the minimum considered clinically significant (3), and yet donanemab’s trial only led to an improvement of around 3 points (4). This means an average person using the drug, or their family, would likely not notice any difference after using the drug for 18 months, despite its claimed successes.
Longer-term impacts have not been tested, and the claimed significant improvement only emerges when we consider the trial participants at a group level.
The drugs also present practical problems. For one, they are not without side effects. Rare but serious side-effects include brain swelling and small strokes, with serious side effects affecting 1.6% of participants in donanemab’s trial, and a much larger 24% developing other less severe reactions to the drug. These drugs are also administered via infusion as an intravenous drip. Patients need to attend a clinic for up to an hour each week to receive the medication, although one new candidate drug – remternetug – is seeking to determine if an injectable alternative is viable, a bit like an EpiPen for diabetics.
Conclusion
Finally, there is a bittersweet result implied by many of the trial results discussed above. These drugs set out to tackle amyloid deposits in the hope that doing so would significantly alter the course of Alzheimer’s disease. They are actually very effective at the physical reduction of amyloids, meaning the muted impacts on patient outcomes are a concern. In other words, we may not have much scope to tweak the drugs to make them better, because they already meet their primary (physical) objectives. Instead, there are likely other entirely separate causes of the disease which we are not yet targeting at all.
These drugs are an important start. They are a proof of concept that Alzheimer’s disease can be modified, and provide some hope for further developments in future. However, those hoping we are on the cusp of turning Alzheimer’s into a manageable disease may be disappointed for a while yet. We will likely need to see significant further progress, including in areas with no successful drugs yet, before we can realistically hope to materially reduce the burden caused by this fearsome disease.
References
- alzint.org
FDA approves Biogen’s aducanumab | Alzheimer’s Disease International (ADI) - FDA Converts Novel Alzheimer’s Disease Treatment to Traditional Approval | FDA
- Integrated Alzheimer’s Disease Rating Scale: Clinically meaningful change estimates - Wessels - 2022 - Alzheimer’s & Dementia: Translational Research & Clinical Interventions - Wiley Online Library
- Meaningful Clinical Changes in Alzheimer Disease Measured With the iADRS and Illustrated Using the Donanemab TRAILBLAZER-ALZ Study Findings | Neurology Clinical Practice
Pacific Life Re Limited (No. 825110) is registered in England and Wales and has its registered office at Tower Bridge House, St Katharine’s Way, London, E1W 1BA. Pacific Life Re Limited is authorised and regulated by the Financial Conduct Authority and Prudential Regulatory Authority in the United Kingdom (Reference Number 202620). The material contained in this booklet is for information purposes only. Pacific Life Re gives no assurance as to the completeness or accuracy of such material and accepts no responsibility for loss occasioned to any person acting or refraining from acting on the basis of such material.
©2024 Pacific Life Re Limited. All rights reserved.

Mike Wilson
Director, Medical Analytics








.png)
.png)




















.png)